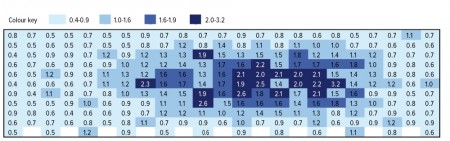
Rapid Non-destructive Moisture Mapping in Freeze Dryers.
Regulatory authorities require proof that lyophilization cycles have been developed logically and demonstrate uniformity. One measure of uniformity is the consistency of residual water content throughout a batch.
.
Generating moisture maps of a commercial freeze dryer is extremely difficult from a practical point-of-view. Traditional moisture analysis methods for pharmaceutical products such as Karl Fischer (KF) titration and thermo-gravimetric analysis (TGA) are very time consuming, involve chemical reagents, and destroy the sample making 100% analysis of a commercial lyo batch difficult if not impossible.
.
The rapid and non-destructive nature of the FMS headspace method makes it an ideal tool to characterize lyophilization cycle efficiency and freeze dryer performance across shelves, between shelves and as a function of drying cycle parameters. Experiments have demonstrated that the amount of headspace water vapor directly correlates to the lyo cake moisture content. Stability studies have shown that the degradation of the active pharmaceutical ingredient correlates to the initial water vapor concentration present in the freeze-dried vial.
.
These results indicate that rapid water vapor determination with an optical method could replace the slow destructive traditional methods for the moisture analysis of freeze-dried product. The ability to perform a useful non-destructive moisture measurement in a matter of seconds means that moisture mapping in a commercial freeze dryer can be done quickly and without loss of product.
.