Rapid non-destructive headspace oxygen monitoring
The need to monitor headspace oxygen levels in parenteral containers arises from the requirement to ensure the stability and potency of oxygen-sensitive product. Besides a loss of efficacy and reduction in shelf life, exposure of such products to oxygen can result in product discoloration, changes in dissolution rate and profile, and even toxicity or other pharmacological properties associated with negative side effects. Headspace oxygen levels are often monitored during the filling process as an in-process control (IPC) of the purging system used to bring headspace oxygen levels below the required specification during filling. A benchtop headspace oxygen analyzer can be set up at-line in production for rapid nondestructive oxygen measurements.
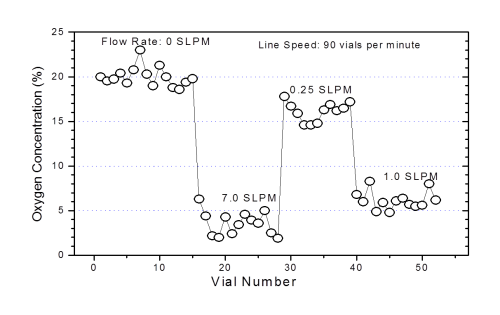
During the set up and validation of a filling line, an at-line oxygen analyzer can be invaluable for the optimization of the purging process. The portability and ease-of-use of the LIGHTHOUSE FMS-Oxygen Headspace Analyzer means that the analyzer can be brought at-line and samples can be immediately analyzed after filling allowing for real time feedback as various parameters on the filling line are changed and optimized. The rapid nature of the measurement means that a large number of samples can be quickly analyzed for the optimization procedure. The fact that the measurement method is non-destructive means there is no need to dispose of destroyed product samples. The savings in time and resource to optimize and validate the filling line can be significant.
Once the line has been optimized and validated, in-process control of headspace oxygen levels during routine production can save significant amount of product by detecting process upsets or by verifying that product still meets specification in the event of a line stoppage.